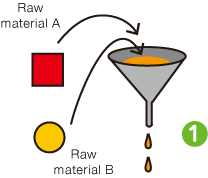
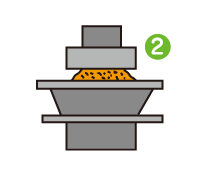
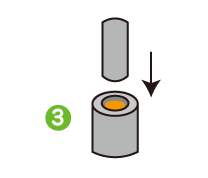
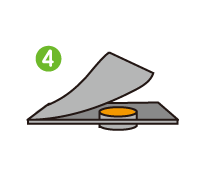
I was surprised to learn that quality management is so meticulously strict that every step of every process is recorded and retained for 10 to 15 years. Incidentally, I used to work in a factory where I continually recorded the results of sample inspections at specified times. From experience, I know that having rules is not the same as sticking to them. You can only make adherence to the rules customary practice by inculcating a proper appreciation of the importance of quality management throughout the workforce.
On entering the next building, dedicated to packaging and final quality assurance, I encounter a memorable scene. A team of robots is busy packaging drugs. Since each robot is in charge of a different process, their shapes and speeds differ. As I watch them work, they look like a band of robot brothers. The largest of the band, applying himself wholeheartedly to his task, must be the oldest. The one so dexterously performing two roles is the second oldest. The one moving more slowly as he neatly places packaged drugs in large boxes is the third oldest. And the one agilely carrying the filled boxes to the specified place is the cheerful baby of the family (chuckle).
People and robots are working together. Robots can perform certain tasks quicker and better than people, but people still manage the robots. Although the division of labor between robots and people is expected to progress, I think people will remain in charge. They will be the ones designing and implementing the quality management and safety control systems and who have the final say.
Listening to an explanation of cost reduction measures to boost competitiveness in the industry, I get the impression that Sumitomo Dainippon Pharma cares deeply about people. My first thought was that cutting labor costs must be high on the agenda. But because the company believes “people are treasures,” it is convinced cost reduction should be achieved by using one’s brain and hands. The workers continually wrestle with the question of what they can do to perform their tasks more efficiently, safely, and accurately. With the aim of making 3,776 proposals for improvement a year (the same number as the height of Mt. Fuji in meters), Suzuka Plant is promoting kaizen (improvement) throughout its operations. Each tiny pharmaceutical tablet from this plant is the fruit of a tremendous amount of effort and ingenuity involving numerous people…… I will reflect on that fact whenever I swallow a pill from now on!

 EN
EN